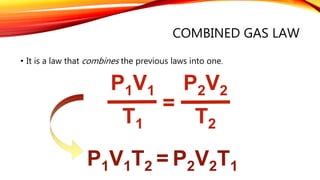
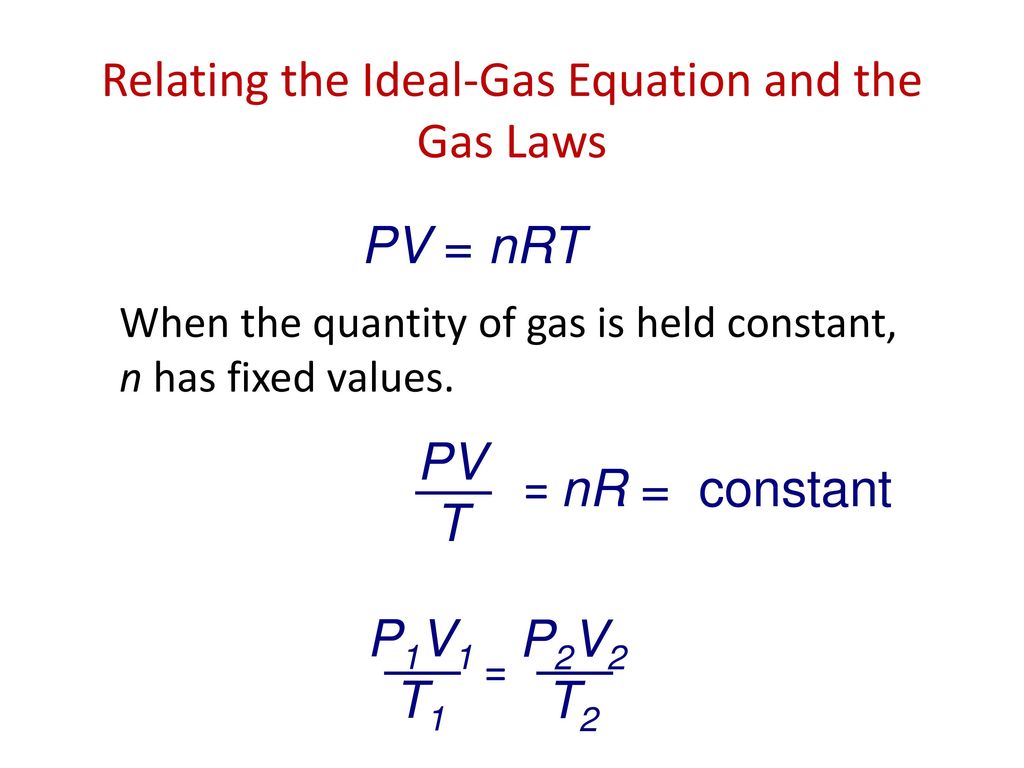
P1V1/T1=P2V2/T2
You can find the volume of CO2 at stp by using the relationship between a mole and volume or that between mass and volume as explained below. Reddit what breaks a fast.
Chapter 10 Gas Laws
Web Nm3h标立米每小时是流体在标准温度和压力下的体积流量国际纯化学及应用化学协会和美国国家标准局都规定STP是指27315 K1 bar80年代前曾以29815 K1 bar或101325 bar为标准.
. Web 标准状态中压力无论国内外都是标准大气压即101325 kPa但是温度就不尽相同我国在用于计算特有气体的质量时有二种温度标准200 用这个的主要作用就是可以对应对出在该温度下空气的密度最终用以计算空气的重量但如果在工业动力用风当中就不需要考虑温度自然也就不需要. Where P1 is initial pressure V1 is initial volume T1 is initial temperature P2 is. Furthermore the conversion to kelvin can easily be done by adding 273 to the particular unit.
The temperature changes from 305K to 32C. The local corner shop stores their 125 L soft drink products on some shelves in a storeroom at the back of the shop. Different between Nm3h m3h.
May flying star 2022. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyles law Charless law. Temperature is when they are both doubled or tripled.
Consider a sample of gas in a rigid closed container at 111 C and 145 atm. T2 T1 x V2V1. Web P1V1T1 P2V2T2.
Which of the following is a correct rearrangement of Charless law. I dont know why this simple fact has escaped modern climate alarmists who seem to attribute lower pressure at higher altitudes with the mysterious fabrication called lapse rate. Any units may be used as long as both the initial and final condition units match.
Web The equation is represented as follows. Web Using the P1V1T1P2V2T2 it is always possible to convert back to the actual conditions that we are required to run the compressor. To keep your business moving forward Brady offers a full range of commercial label printers and industrial label printers including the BradyPrinter i3300 BradyPrinter i5100 BradyPrinter i5300 BradyPrinter i7100 and the Wraptor.
If we know that P2 03P1 and we compare equal volumes of air it follows that T2 should be around 03T1. Web 公式P1V1T1P2V2T2 Nm3h 是在 0 度或 20 度1atm 下的标准流量后者是在工作温 度及工作压力下的流量. We need to convert to Kpa.
The weather forecast for the coming weekend is said to reach temperatures of 40. Basic Lab Techniques Equipment. Web Help your students classify ideas and communicate more effectively with these free graphic organizer templates available for download.
What will be the pressure of the gas in atm at 150 C. When it rises to an elevation where the pressure is 0720 atm. Web Answer 1 of 6.
An important point to note is that the temperature should always be in kelvin for the purpose of calculation. 1 mole of CO2 or any gas occupies 224 dm3 at stp where 224 dm3 is called molar volume of a gas at stp. Web P1V1T1 P2V2T2.
There is increase In pressure from P1 to P2. The 224 Litersmole quantity can be derived from the Ideal Gas Law PV nRT plugging in STP conditions for P and T and solving for Vn which gets 224 Litersmole. At STP a mole of gas takes up 224 Liters.
The difference between Nm3h and m3h for compressor is very important because the first one correspond to standard conditions 1 bar and 20C where the density of the air is 12060 kgm3. Web 1定义 Nm3h指标准立方米每小时扩展 Nm3 Nm3是指在0摄氏度1个标准大气压下的气体体积N代表名义工况Nominal Condition即空气的条件为一个标准大气压 温度为 0C 相对湿度为0 m3是指实际工作状态下气体体积 Sm3是指对照条件下可参考条件下一般是20摄氏度1 个标准大气压. Web P1V1T1P2V2T2 V1V2 P2P1 T1T2.
Web P1V1T1 P2V2T2. Web The scenario where volume doesnt change with changes in pressure and. The local corner shop stores their 125 L soft drink products on some shelves in a storeroom at the back of the shop.
Web P1V1 T1 P2V2 T2 Using this knowledge and the results from the experiment apply what you have learnt to the following scenario. Web Nm3是指在0摄氏度1个标准大气压下的气体体积N代表标准条件即空气的条件为一个标准大气压 温度为 0 相对湿度为0. So if the units are available in the Celsius scale then one must convert them to kelvin.
The combined gas law is shown below. Web The ideal gas law also called the general gas equation is the equation of state of a hypothetical ideal gasIt is a good approximation of the behavior of many gases under many conditions although it has several limitations. To find the exact volume of your gas it depends on what informati.
Web 2016-05-30 阿伏伽德罗常数公式 2018-10-19 阿伏伽德罗常数的计算急需. M3h usually correspond to the flow at pressure conditions 7 bar and 60C where the density of the. They can be used to structure writing projects and help in problem solving decision making studying planning research and brainstorming.
V1V2 202101310155 V1V24. The weather forecast for the coming weekend is said to reach temperatures of 40. 2009-01-16 阿伏伽德罗常数计算公式的推导过程 2010-09-12 阿伏伽德罗常数怎么计算 585 2019-10-22 阿伏伽德罗常数是怎么计算出来的 11 2014-05-11 化学关于阿伏伽德罗常数的计算 2013-10-08 阿伏伽德罗常数是怎么计算出来的.
Metric ruler balance 100 mL beaker 10 mL graduated cylinder 100 mL graduated cylinder Intro to Chemistry Equipment. Web Nm3h标立米每小时是流体在标准温度和压力standard temperature and pressureSTP下的体积流量国际纯化学及应用化学协会IUPAC和美国国家标准局NIST都规定STP是指27315 K1 bar80年代前曾以29815 K1 bar或101325 bar为标准. Therefore the volume has decreased by factor of 4.
So for example the compressor manufacturer will test the compressor at the actual conditions there but will specify the results at the agreed reference conditions to avoid ambiguities. A tube closed at one end and marked in units of milliliters. Web P1V1 T1 P2V2 T2 Using this knowledge and the results from the experiment apply what you have learnt to the following scenario.
Web 高中物理网课平台哪个好 受欢迎的是哪个. What units can be used when performing a Boyles law calculation. Final pressure V2 is final volume and T2 is final temperature.
So depending on the. Web Answer 1 of 8. A 120 L weather balloon on the ground has a temperature of 250C and is at atmospheric pressure 100 atm.

Boyle S Law Practice Problems Examples Explained P1v1 P2v2 Youtube
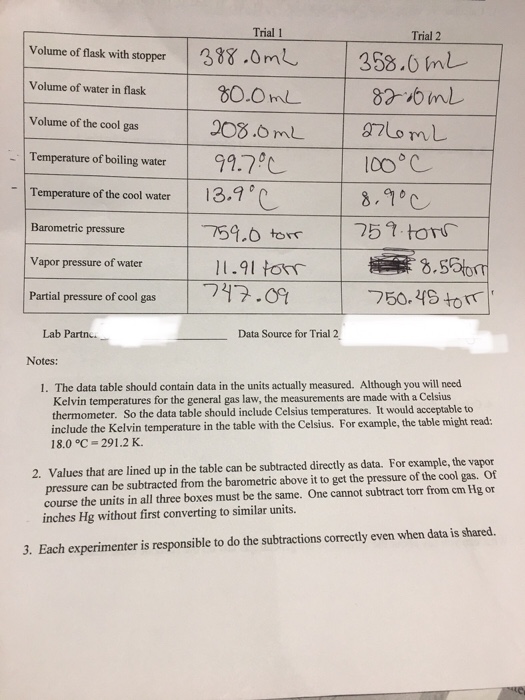
Solved The Questions Are Based Upon The General Gas Law Of Chegg Com

Gas Laws Ppt Download
Deduce General Gas Equation P1v1 T1 P2v2 T2 Sarthaks Econnect Largest Online Education Community
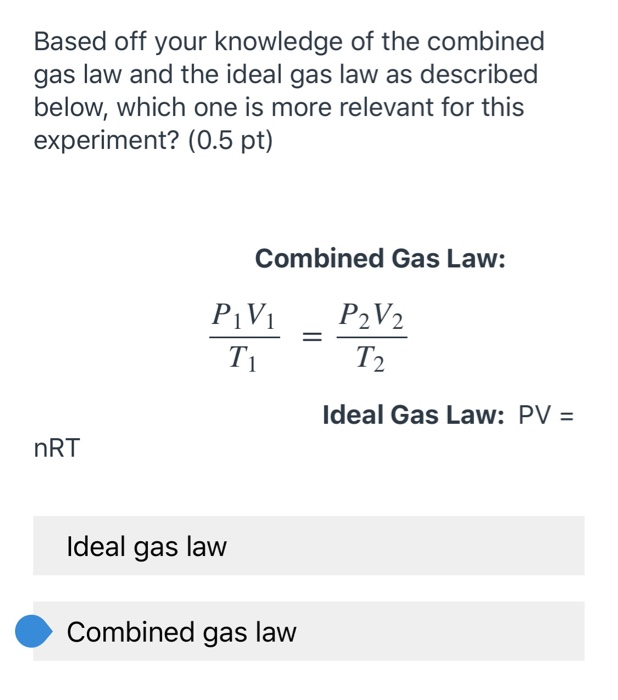
Solved Based Off Your Knowledge Of The Combined Gas Law And Chegg Com
Solved Uestion 11 Of 20 Gt A Sample Of An Ideal Gas Has A Volume Of 2 20 Course Hero

Combined Gas Law Tutorial V1p1 T1 V2p2 T2 Youtube

Combined Gas Law P1v1 T1 P2v2 T2 Examples Practice Problems Calculations Equation Youtube
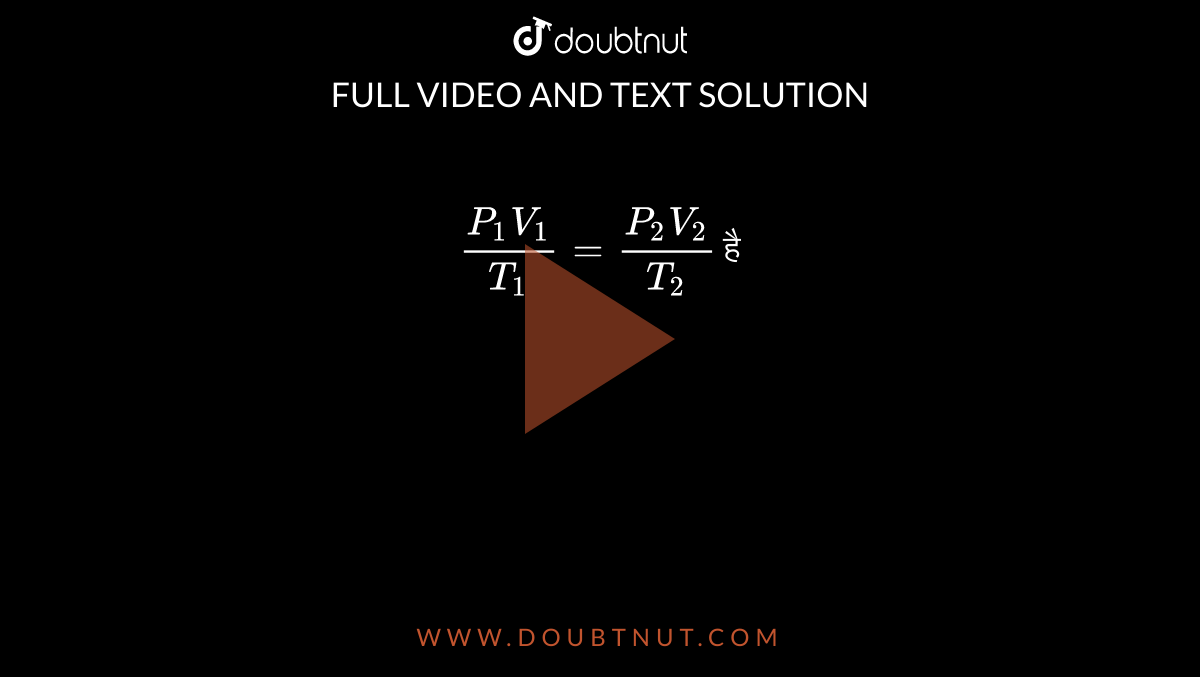
State Boyle S And Charles S Law Use These Laws To Derive The Relationship P1v1 T1 P2v2 T2
Unit 7 Gas Laws Ms Roman S Chemistry Page

When To Use The Concept Pv Constant And P1v1 P2v2 To Solve Evan S Space

Ley General Youtube

Equacao Geral Dos Gases Exercicios Resolvidos P1 V1 T1 P2 V2 T2 Youtube

Air Standard Cycle P V Diagram And Thermal Efficiency

Combined Gas Law Formula Definition Concepts And Examples

Media Portfolio
Gases Ppt Download